Petrochemicals
Collaborations with customers
AKO Yoga
Namasté with AKO YOGA & Hexamoll® DINCH
Yoga strengthens your muscles, reduces stress and trains your attention. But most important – it’s a lot of fun! To get the most out of your yoga sessions, you need a high-quality yoga mat: if they are made from PVC, they show the longest durability.
A. Kolckmann GmbH is a long-term customer of BASF and uses Hexamoll® DINCH in their PVC yoga mats of the AKO YOGA line. They produce their mats in Alfdorf in Southern Germany. With AKO YOGA, yogis get non-slip mats with a stable surface, good grip, and excellent cushioning qualities, which yet remain flexible and pleasantly soft. The mats come in a variety of thicknesses and designs.
But one of the key aspects is that the yoga mats are certified with OEKO-TEX® Standard 100 Class 1. This class also includes articles for babies and toddlers and is the highest achievable standard for textile products. Hexamoll® DINCH contributed to this certification as a raw material that adheres to strict rules and is suitable to be an ingredient in textiles (product classes I to IV) that comply with the strict requirements of OEKO-TEX® Standard 100.
Hexamoll® DINCH is the trusted non-phthalate plasticizer especially developed for applications with close human contact: Besides yoga mats, Hexamoll® DINCH is also ideal for medical devices, children’s toys or food contact materials as it has an excellent toxicological profile and is approved and certified by many authorities and institutions worldwide.
Cornelius Sorg, managing director of A. Kolckmann GmbH, underlines that Hexamoll® DINCH’s suitability for OEKO-TEX® Standard 100 compliant textiles is one of their main motivations to use this plasticizer. They even developed their product portfolio further and introduced baby mats with Hexamoll® DINCH.

Quality is important for A. Kolckmann GmbH and Cornelius Sorg further explains: “We continuously improved over time and in this context replaced our previous plasticizer with Hexamoll® DINCH, which is the perfect solution for us. We do not need a lot of plasticizers in the formulation, but we are able to achieve a soft mat that fulfills our customers’ requirements. In fact, we also tested alternative non-phthalate plasticizers, but we decided to use BASF’s Hexamoll® DINCH not only because of the product properties but also because of the technical support of BASF. The employees assisted us in adjusting our formulation and we very much value the expertise, which BASF offers us with their highly skilled technical marketing team and own laboratory.”
We at BASF are happy to partner with AKO YOGA!
Interested in more information about AKO YOGA? Click here.
Image: A. Kolckmann GmbH
Bain Medical
Bain Medical & Hexamoll® DINCH - World Kidney Day
Bain Medical Equipment (Guangzhou) Co., Ltd. and Hexamoll® DINCH from BASF contribute to the best possible treatment for chronic kidney disease patients. Let’s find out how!
Bain Medical was established in China in 2003 and has become a leading manufacturer of medical equipment for blood purification. The company is committed to research and development, manufacturing and sales of devices and disposables for blood purification, which mainly help kidney disease patients worldwide.
One potential therapy is hemodialysis. It is one of the replacement therapies for acute and chronic renal failure. During the treatment, blood is drawn out through an extracorporeal blood circuit and it flows through the dialyzer. Within the dialyzer, extracorporeal removal of waste products takes place and the blood is then pumped back into the human body.
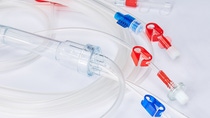
Bain Medical manufactures various parts for this therapy, e. g. the extracorporeal blood circuits. They need to be flexible, which makes PVC the material of choice. And as they are in touch with the blood that is re-directed into the body, it is of greatest importance that the material is suitable for this application. That’s why Bain Medical uses Hexamoll® DINCH from BASF in the extracorporeal blood circuits.
Hexamoll® DINCH is BASF’s trusted non-phthalate plasticizer especially developed for applications with close human contact: medical devices are one application area, but Hexamoll® DINCH is also ideal for children’s toys or food contact materials as it has an excellent toxicological profile and is approved and certified by many authorities and institutions worldwide.
Dr. Yu Liu, R&D Director of Bain Medical, explains why they chose Hexamoll® DINCH from BASF: “Customer safety is of utmost importance to us. Therefore, one key reason we chose Hexamoll® DINCH from BASF was that scientific studies prove its suitability even for blood storage1. It more so shows advantages compared to other products: Hexamoll® DINCH demonstrates lower migration rates than DEHP (DOP) (a phthalate plasticizer still dominant in the market).”
That means less plasticizer is taken up by the blood and ultimately by the human body. In addition to this scientific argument, BASF’s regulatory compliance is another major reason why Bain Medical uses Hexamoll® DINCH. “It is included in the European Pharmacopoeia, which provides common quality standards for medical devices and pharmaceutical products”, explains Dr. Liu. “By selecting a product, that is listed in there, we can ensure patients’ safety.”
For a medical device to be approved for patients, it must undergo a complex process, which differs by country and aims at ensuring safe applications for patients. In China, manufacturers of medical devices need a company-specific approval of the medical device by the National Medical Products Administration (NMPA). This requires intensive documentation in various languages, covering e. g. toxicological data, ingredient properties, application details and details on formulation.
Bain Medical highly appreciates BASF’s regulatory expertise. “The dedicated regulatory team at BASF helped us to prepare all the documents needed for the National Medical Products Administration. With their support, we could introduce several new products with Hexamoll® DINCH into the market over the past years. Therefore, we would like to thank them very much!”
We at BASF highly value the partnership with Bain Medical to ensure high quality medical devices. It allows us to jointly contribute to future-oriented products.
Image: BASF SE
1 Zhong et al.: In vitro investigation of the effect of plasticizers on the blood compatibility of medical grade plasticized poly (vinyl chloride), Springer Science+Business Media, New York 2013.
Prowse et al.: Commercially available blood storage containers, The International Journal of Transfusion Medicine, International Society of Blood Transfusion, 2013.
Bicalho et al.: Blood Bag Plasticizers Influence Red Blood Cell Vesiculation Rate without Altering the Lipid Composition of the Vesicles, Transfusion Medicine and Hemotherapy, 2015.
Lagerberg et al.: In vitro evaluation of the quality of blood products collected and stored in systems completely free of di(2-ethylhexyl)phthalate–plasticized materials, Transfusion, Volume 55, March 2015.
